Viral Vectors for Gene Transfer
Viral vectors are popular research tools in biological sciences and other fields. They are customizable, allowing for research with different genes of interest in a variety of in vitro and in vivo applications. Viral vectors are used to deliver genes to cells. They can either add a gene (knock-in) to study the function of a particular gene or to delete a gene (knock-down) to study the effects of gene deletion or reduction.
All work with viral vectors is subject to the NIH Guidelines for Recombinant or Synthetic Nucleic Acids, and as such, all work with viral vectors must be approved at a convened Institutional Biosafety Committee (IBC) meeting. EH&S Biological Safety along with the IBC will perform a risk assessment for the vector system and gene inserts. If you wish to add work with viral vectors, refer to Biological Research Approval for more information. The most commonly used viral vectors are described here, and the table below lists the biosafety levels required at the University of Washington.
Biosafety Levels for Viral Vectors
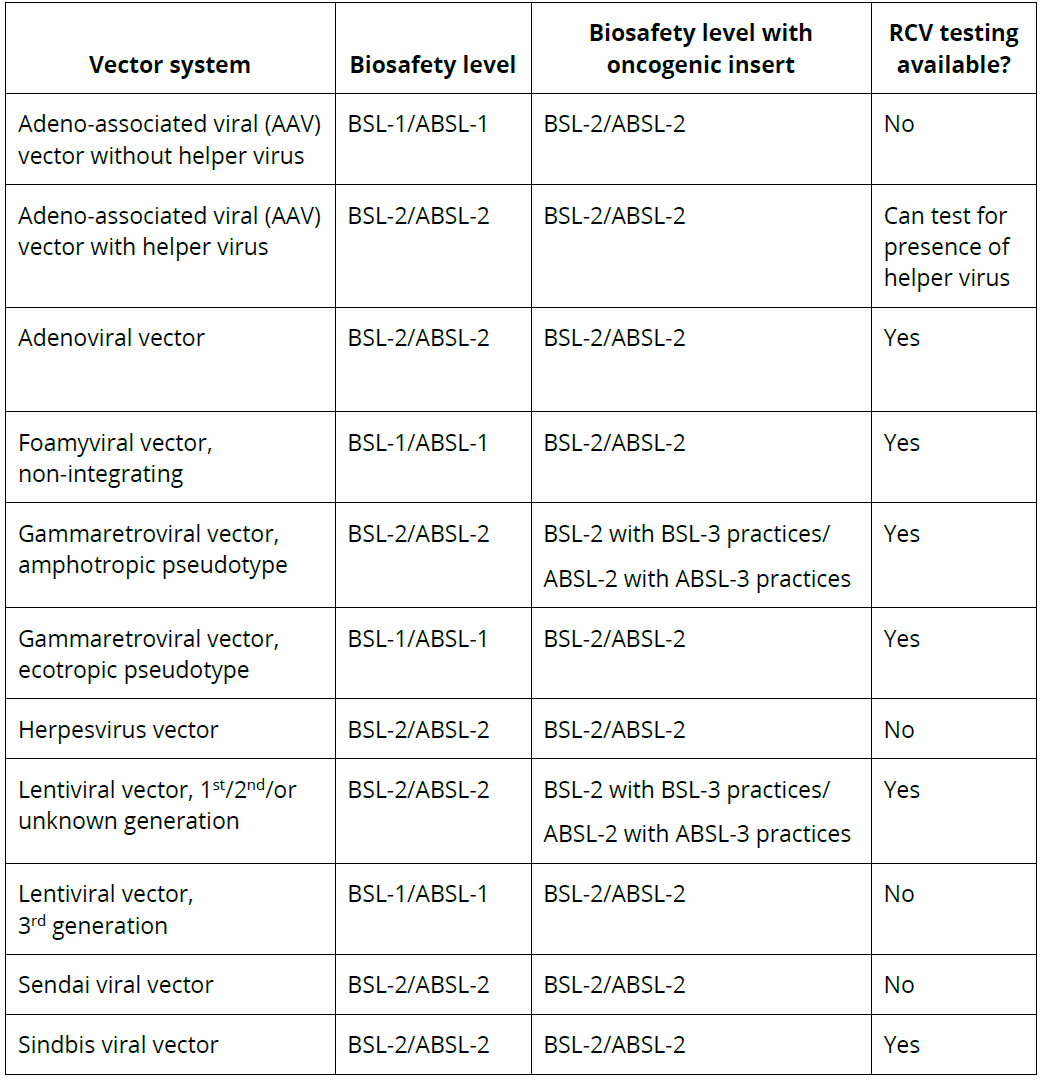
The following table lists the biosafety levels (BSLs) for viral vector systems with and without oncogenic inserts. For RCV testing information, refer to specific information for each vector. Click on the table to access the PDF.
Adeno-associated viral (AAV) vectors
- AAV are small non-enveloped viruses with single-stranded DNA (ssDNA) genomes of approximately 4-5 kb.
- AAV rely on gene products provided by co-infection with other DNA viruses, mainly adenoviruses or herpesviruses, to complete their life cycle. In virus-free AAV production systems, these genes are provided on plasmids.
- No human disease has been associated with AAV infection.
- AAV types 1-9 have been developed as gene delivery vectors, including for use in human gene transfer studies.
- There may be risks associated with the function of the transgenes used (i.e., toxins or oncogenes).
- Wild type AAVs integrate into the host chromosome, but the vectors do not retain integrative functions. Nonetheless, the presence of linear DNA in the genome can lead to spurious integration through DNA repair mechanisms, particularly if there is substantial homology with host genes.
For AAV without helper virus:
- AAV vector stocks generated with adenovirus-free packaging systems can be handled at BSL-1, and no further testing is needed.
For AAV with helper virus:
- Test for the presence of replication-competent adenovirus in the purified AAV stock.
Adenoviral vectors
- Adenoviruses are non-enveloped viruses with double-stranded DNA (dsDNA) genomes of approximately 36 kb.
- There are over 50 recognized serotypes of human adenoviruses (HAdVs) causing a range of diseases including respiratory, ocular, renal, and gastrointestinal.
- HAdV-5 based vectors are most commonly used for routine gene transfer.
- Canine, mouse, and simian adenoviruses also commonly used.
- Categories of replication-defective adenoviral vectors:
- First generation – The transgene is inserted in place of the E1 transcription unit, which restricts replication to cells capable of trans-complementing E1, such as HEK-293 cells.
- Second generation – These vectors contain additional deletions or mutations in other early transcription units (E2 or E4) to further limit viral gene expression.
- Third generation – Called “gutless” or “helper-dependent,” these vectors have all of the viral genes replaced with heterologous DNA and require the use of a helper virus during production in order to package the vector DNA.
- There may be risks associated with the function of the transgenes used (i.e., toxins or oncogenes).
- Possible reversion of vectors to replication-competence during production or as a consequence of recombination with wild type viruses. Replication competent virus (RCV) testing can be performed after production.
- Because adenoviruses are highly immunogenic, a robust immune response to the vector can be dose-limiting for human or animal applications.
- For first generation vectors, the stock should be tested by PCR for E1 using an assay sufficiently sensitive to detect 1 RCV in 3 x 10^10 virus particles. A known positive control demonstrating this sensitivity and the results of the test for each vector stock must be available upon request.
- A similar test is adequate for third generation vectors.
Gammaretroviral vectors
- Gammaretroviruses are enveloped viruses with RNA genomes.
- They are notable for the activity of the virus-encoded reverse transcriptase, which converts the RNA genome into a DNA copy during cell entry. The DNA copy of the genome, or provirus, is integrated into the host chromosome and becomes a stable part of the host genome.
- Gammaretrovirus is a genus of Retroviridae that includes common vectors based on murine leukemia viruses (MLVs) and Gibbon ape leukemia virus.
- MLVs are further subdivided into classes based on tropism, which is dictated by envelope glycoprotein-receptor interactions. The two classes most relevant for vectors are:
- Ecotropic – narrow tropism for mouse or rat cells
- Amphotropic – broad tropism for rodent cells as well as other cell types, including human
- In many cases, the native envelope glycoprotein is not used. Rather, an envelope glycoprotein from a virus unrelated to the rest of the genome [e.g., the G glycoprotein from vesicular stomatitis virus (VSV-G)] is incorporated into the viral vector particles. This is called pseudotyping.
- There may be risks associated with the function of the transgenes used (i.e., toxins or oncogenes).
- Amphotropic vectors with oncogenic inserts require an increased biosafety level.
- There is a risk of reconstitution of replication competent virus (RCV) during vector production and use.
- Insertional mutagenesis could occur during integration of the provirus. Any retroviral integration has the potential to disrupt a critical gene function or de-regulate expression of cellular genes. Once integrated, the transgene will be present for the lifetime of the transduced cell.
- Gammaretroviral vectors with pseudotypes other than ecotropic must be tested for RCV by a marker rescue assay prior to being approved for use at a lower biosafety level.
- The vector stock or producer line should be tested at a limit of sensitivity of 1 infectious unit/mL and should include a known positive control.
- Reference: Wilson, C.A., Ng, T. H., and Miller, A. E., 1997. Evaluation of recommendations for replication-competent retrovirus testing associated with use of retroviral vectors. Human Gene Therapy, 8(7): 869-874.
Lentiviral vectors
- Lentiviruses are enveloped viruses with RNA genomes.
- They are notable for the activity of the virus-encoded reverse transcriptase, which converts the RNA genome into a DNA copy during cell entry. The DNA copy of the genome, or provirus, is integrated into the host chromosome and becomes a stable part of the host genome.
- Lentivirus is a genus of Retroviridae that includes human immunodeficiency viruses (HIV)-1 and -2, simian immunodeficiency virus (SIV), and feline immunodeficiency virus (FIV).
- Generations of lentiviral vectors:
- First and second generation vectors: The initial strategy in vector development was the creation of a three-plasmid system whereby the genome lacking the envelope gene (env) and a packing sequence is encoded on one plasmid, env is encoded on a second plasmid, and the gene of interest is encoded by a third plasmid containing the viral long terminal repeats (LTRs) and a functional packaging sequence. Second generation vectors removed additional virulence genes from plasmid one that were unnecessary for vector function.
- Third and fourth generation vectors: Third generation vectors moved rev to a separate plasmid. Fourth generation vectors split gag and pol onto separate plasmids, while further refining the viral genes that are expressed to enhance vector function. Third and fourth generation vectors also commonly employ self-inactivating (SIN) sequences that reduce expression from the viral LTRs.
- In most cases, the native envelope is not used. Rather, an envelope glycoprotein from a virus unrelated to the rest of the genome [e.g., the G glycoprotein from vesicular stomatitis virus (VSV-G)] is expressed from the second plasmid, called pseudotyping. The plasmids are co-transfected into a cell that then produces the viral vector. The particle is composed of proteins from the helper plasmids, but only the nucleic acid from the plasmid encoding the gene of interest is encapsidated. Thus, the vector is not replication-competent.
- There may be risks associated with the function of the transgenes used (i.e., toxins or oncogenes).
- There is a risk of reconstitution of replication competent virus (RCV) during vector production and use.
- Insertional mutagenesis could occur during integration of the provirus. Any retroviral integration has the potential to disrupt a critical gene function or de-regulate expression of cellular genes. Once integrated, the transgene will be present for the lifetime of the transduced cell.
- Lentiviral vector stocks generated with 1st and 2nd generation packaging systems devoid of the HIV envelope gene must be tested for RCV by serial transfer in a cell line documented to be capable of supporting wildtype HIV and ELISA assay for p24 antigen prior to approval for use at a lower biosafety level.
- The vector stock should be tested at a limit of sensitivity of 1 infectious unit/mL. Test a volume of at least 1 mL (or equivalent if viral stock is concentrated).
- Lentiviral vector systems with HIV envelope sequences require BSL-2 with BSL-3 practices at a minimum.
- The risk of RCV reconstitution for third and fourth generation lentiviral vectors that also employ SIN LTRs is so low that the UW IBC has determined that RCV testing is not required for vectors to meet this definition. Refer to Third Generation Lentiviral Vectors.
What you need to know
- Biological Use Authorization (BUA) is required for all research involving recombinant or synthetic nucleic acids (DNA/RNA).
- Submit a BUA application or BUA change application to initiate EH&S and IBC review for work with viral vectors.
- Use of viral vectors in animals requires IACUC approval.
Frequently asked question
For most of the commonly used viral vectors, a freshly prepared 1:10 dilution of household bleach is a highly effective disinfectant. You can also choose a disinfectant from the EPA’s Registered Antimicrobial Products Effective Against Bloodborne Pathogens. These are effective against bloodborne pathogens and will also decontaminate most viral vectors. If you have questions about choosing a disinfectant for your work, please contact EH&S Biological Safety at ehsbio@uw.edu.